OUR WAREHOUSE
I.V.A.S. WAREHOUSING



The Warehouse
Features:
Our warehouse covers an area of 55,000 square meters, with a covered area of 12,000 square meters; this allows for temperature-controlled storage at 15-25°C for up to 22,000 pallets.
The warehouse is monitored 24 hours a day by specialized personnel to ensure the maximum security of your goods, and all access points are controlled.
The warehouse is equipped with 12 automatic docks for loading and unloading heavy vehicles, a side loading area, and a large parking lot equipped with restrooms and a break area for waiting drivers.
Equipped with the best and most modern security systems, the building was designed with the concept of a “smart” warehouse: home automation allows the monitoring of various parameters (from LED lighting to the recharging of service vehicles), with the aim of using only the necessary energy and avoiding unnecessary waste, a common issue in all traditional warehouses.
Temperature Control
The storage of pharmaceutical products requires precise environmental conditions.
Our warehouse is equipped with advanced temperature control systems that maintain constant storage conditions to ensure the integrity of the products.
This is particularly crucial for temperature-sensitive drugs, such as vaccines and other biotech products.

Computer Systems Associated with Thermal Control
Our software has been validated and is fully compliant with CFR 21 Part 11 regulations.
CFR 21 Part 11: Ensures that our electronic systems and electronic signatures are reliable and equivalent to traditional paper signatures. This compliance guarantees the security, integrity, and traceability of electronic data, which is essential for companies operating in the pharmaceutical, biotech, and medical fields.

Goods Management System: Operational Efficiency
Our goods management system (ERP) is validated to ensure maximum traceability and accuracy. This automated system handles all stages of the logistics process, from goods intake to their dispatch, reducing human errors and enhancing operational efficiency. Every stock movement is recorded in real-time, allowing for precise inventory control.
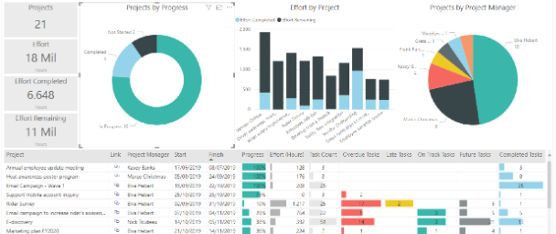
Power BI
Our graphical interface enables the customer to stay connected to our computer system, independently accessing all real-time inventory details. The process of opening complaints and deviations is made even more functional and integrated through the use of the same customer interface.
Internal Audits
Our internal audits are designed to continuously monitor and improve business processes. These audits identify areas for improvement, verify compliance with regulations, and ensure that all operations are conducted according to the highest standards of quality and safety. Recommendations resulting from audits are implemented to continuously optimize our operations.

Change Control
We have a robust change control system in place to manage and document any modifications to operational processes. This system ensures that all changes are evaluated, approved, and adequately communicated before implementation, minimizing risks and ensuring operational continuity without compromising product quality and safety.

Quality Assurance
The functions of QA (Quality Assurance) and QP (Qualified Person) are integrated into our operational process. The QA and QP team is responsible for ensuring that all operations comply with GMP (Good Manufacturing Practices) and GDP (Good Distribution Practices) regulations. This includes overseeing procedures, managing documentation, and conducting regular internal audits.
Quarantine Management
We implement rigorous quarantine procedures to isolate and manage non-conforming or suspicious products until issues are resolved. Quarantine areas are separate from main storage spaces and continuously monitored. This ensures that non-conforming products do not enter the distribution cycle, thereby protecting consumer health and our clients’ reputation.
Ongoing Certifications
The certifications currently being pursued are:
– GMP Authorization (AIFA)
– GDP Certification
– ISO 9001/2015 Certification
– Authorization 219/06 for the distribution of medicinal products for human use (ATS)
– Authorization 193/06 for the distribution of medicinal products for veterinary use (ATS)
– Narcotics Storage Authorization (Ministry of Health)
– PMDA Accreditation
– FDA Registration

Phone
+39 342 6309069
Request information
Location
Sede Legale: Via Leone Pancaldo 6, 20129 Milano (MI)
Sede Operativa: Strada Provinciale 161 (ex via Molina) n°46, 20052 Vignate (MI)
